Second Edition of the Textbook
The Front Cover Picture's Message to Principles of Physical Chemistry
A Comment by Hans Kuhn and Horst-Dieter Försterling
The picture symbolizes the importance of inventing simplifying models to treat complex phenomena. Such models are crucial in developing the modern topics considered in this book, and we want to emphasize their potential in future research and development.
Modeling a randomly coiled molecule by a dumb-bell (the cover image) was suggested by Werner Kuhn to Hans Kuhn when he began to work for his doctorate, investigating decoiling in a flowing viscous solvent. HK was fascinated by the model's simplicity and by its great success in theoretically analyzing a broad variety of experiments in quantitative terms. This experience and his postdoctoral work with Linus Pauling and Niels Bohr, supported this fascination for powerful simple models and was determining for his life's work in research.
Horst-Dieter Försterling and Hans Kuhn cooperated for many years in research and teaching and from this collaboration the idea grew to write a new kind of textbook on physical chemistry. A textbook that would transmit the thinking process that is essential for performing research. A process that HK had learned from his great teachers: their intuitive approach to scientific questions, their ability to discern the essential from the nonessential, and their independent ways of thinking, having in mind the broad scope and coherence of physical chemistry.
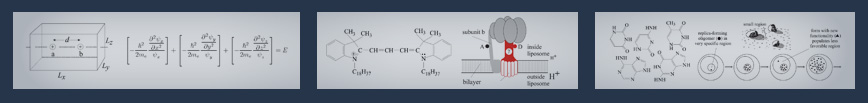
Another instructive model based on this way of thinking is the particle-in-a-box model. It is used to describe the basic behavior of electrons in molecules. In this book it is a touchstone illustrating its value and its limits.
It is fun to see how the box model arose. In Pauling's lab HK was trying to understand the color of polyenes by describing &pi-electrons as particles in a box and he was greatly disappointed - it did not work. Later, when applying the box-model to cyanine dyes he observed a quantitative agreement with experiment. The reason why he had failed in polyenes: an instability leading to an alternation between single- and double-bonds. Considering this instability the box-model had to be slightly improved and then the fundamental difference between the &pi-electron distribution of a polyene and a cyanine was explained. The box-model and its improvements developed into a theory on the light absorption of organic dyes. The particular properties of conducting polymers are based on the theoretical relation between bond alternation and equalization. Both items are discussed in this book, showing the power of a simple model.
The box-model for &pi-electron systems was approximated considering only the component along the molecular chain by the standing waves of a vibrating string. A branched &pi-electron system was then intuitively handled by the standing waves of a branched vibrating string. In searching for a deeper understanding of this simple model, Niels Bohr, exposed to the problem, gave the splendid advice: "solve the 3-dimensional Schrödinger equation for a branched box ".
Inventing simplifying models is the basis in attempting to construct supramolecular machines, stimulated by the revolutionary change in molecular biology. The idea was expressed in the early 1960ies that preparative chemistry should have a new goal: fabricating useful molecular machines by synthesizing different kinds of molecules in a planned manner to precisely interlock and interact purposefully. Simple prototypes were realized as described in the book. This new paradigm in chemistry is strongly developing today termed as supramolecular chemistry, molecular electronics and systems chemistry.
The origin of life is understood as an important new topic in physical chemistry, beginning with the search for a basic theoretical understanding of why and how that kind of a process can take place. The way to approach this fundamental problem is described in the book: inventing a sequence of reasonable steps, each driven by a very particular and particularly changing environment leading to systems with a life-like genetic apparatus. Each step constitutes a simple model as symbolized by the front picture. The emergence of life is closely related to the above mentioned paradigm: constructing supramolecular machines. The skill of the experimentalist is replaced in life's origin by very particular conditions given by chance in a very particular small location on the prebiotic earth.
The Back Cover to Principles of Physical Chemistry
"This admirable text provides a solid foundation in the fundamentals of physical chemistry including quantum mechanics and statistical mechanics/thermodynamics. The presentation assists the students in developing an intuitive understanding of the subjects as well as skill in quantitative manipulations. Particularly exciting is the treatment of larger molecular systems. With a firm but gentle hand, the student is led to several organized molecular assemblies including supramolecular systems and models of the origin of life. By learning of some of the most productive areas of current chemical research, the student may see the discipline as an active, young science in addition to its many accomplishments of earlier years. This text makes physical chemistry fun and demonstrates why so many find it a stimulating and rewarding profession."
Professor Edel Wasserman, President (1999) of the American Chemical Society
Principles of Physical Chemistry takes readers from atoms to increasingly complex molecular assemblies including natural and artificial supramolecular machines
Principles of Physical Chemistry presents a novel approach to physical chemistry that emphasizes the use of a few fundamental principles to quantitatively describe the nature of molecules and their assemblies. It begins with atoms and molecules, using the electron-in-a-box model to illustrate the essential features of quantum mechanics and why atoms and molecules exist. Thermodynamics is not introduced in the classical manner, considering the first and second law as postulates, but approached by studying assemblies of molecules statistically. The authors proceed to molecular assemblies of increasing complexity, evolving from ideal gases to real gases and solutions, then to macromolecules and supramolecular machines, and ending with the search for the logical conditions and chemical requirements for physicochemical processes leading to life's origin, the emergence of matter that carries information. This text is ideal for both undergraduate and graduate courses in physical chemistry. providing a basis for understanding the nature of chemical processes in biology, chemistry, and engineering.
Principles of Physical Chemistry examines several important topics that are often overlooked, yet are critical to a full understanding of the field, including:
- Macromolecules
- Principles of organized molecular assemblies
- Construction of supramolecular machines
- Basic mechanisms in the emergence of information producing and carrying forms of matter
Throughout the text, actual experimental data are used to help readers understand the practical implications of theoretical developments. Simple physical models and examples are used to explain molecular and supramolecular systems and processes. The CD-ROM packaged with the text offers problems, exercises, interactive Mathcad exercises and data tables with search functions that enable readers to apply their newfound skills and knowledge to solving actual problems. In addition, the CD contains Foundations and Justifications, in which mathematical proofs and derivations are presented.
Contents to Principles of Physical Chemistry:
- Preface
- Wave-Particle Duality
- Essential Aspects of Structure and Bonding
- Schrödinger Equation
- Hydrogen Atom
- Atoms and the Variational Principle
- A Quantitative View of Chemical Bonding
- Bonding Described by Electron Pairs and Molecular Orbitals
- Molecules with &pi-Electron Systems
- Absorption of Light
- Emission of Light
- Nuclei: Particle and Wave Properties
- Nuclear Spin
- Solids and Intermolecular Forces
- Thermal Motion of Molecules
- Energy Distribution in Molecular Assemblies
- Work w, Heat q, and Internal Energy U
- Reversible Work wrev, Reversible Heat qrev, and Entropy S
- General Conditions for Spontaneity and its Application to Equilibria of Ideal Gases and Dilute Solutions
- Formal Thermodynamics and its Application to Phase Equilibria
- Real Gases
- Real Solutions
- Reaction Equilibria in Aqueous Solutions and Biosystems
- Chemical Reactions in Electrochemical Cells
- Chemical Kinetics
- Transition States and Chemical Reactions
- Macromolecules
- Organized Molecular Assemblies
- Supramolecular Machines
- Origin of Life: Matter Carrying Information